A CEREBRIU APOLLO™ SOFTWARE SUITE ENABLED CONCEPT
By Dr Michel Nemery
Head Of Clinical Development at Cerebriu
Former Chair Of Radiology
The concern over growing reporting backlogs for outpatients is becoming increasingly evident. As demographic shifts and technological advancements lead to a rise in MR imaging volumes*, the strain on a limited number of radiologists is intensifying.
Notably, the surge in MRI requests related to Alzheimer’s disease -requiring relatively high-priority reporting – further affects the management of these backlogs. This confluence of factors underscores the urgent need for innovative solutions to address the challenges faced in radiology today.

THE CONCEPT OF SAFE REPORTING BACKLOG IN MRI EXAMINATIONS
The concept of a “safe reporting backlog” center on optimizing safety, prioritization, and quality of care for patients who have completed an MRI examination while awaiting final interpretation and reporting by an expert radiologist.
A backlog of MRI exams for reading and reporting can be anything from hours to days and weeks – influenced by local workflows, patient case-mix and the volume of scans being processed. For instance, an outpatient who undergoes an MRI scan at 6 PM may not have their images reviewed until the following day at noon. Similarly, patients scanned on Friday afternoons might experience delays in reporting until the following Monday or even later.
The implementation of streamlined workflows and advanced technologies, such as artificial intelligence, enables healthcare providers to enhance the triaging process and prioritize critical cases for immediate review. This approach mitigates the risks associated with extended wait times and significantly improves patient outcomes through more timely and effective care.
STREAMLINING REPORTING WITH APOLLO™
The implementation of Apollo* can facilitate the effective management of reporting backlogs by enabling the prompt and safe screening of all pertinent brain MRIs using artificial intelligence immediately after the sequences and scans are completed. The system is designed to flag critical and clinically significant findings : intracranial tumors, hemorrhages, and acute or subacute infarcts.
Once the brain MRI scan data are sent from the Picture Archiving and Communication System (PACS) to the Apollo™ server, Smart Priority performs inference and detection of these clinical findings. Subsequently, the studies are triaged and organized into a Smart Reading worklist for the reading radiologist. These processes are fully automated, enhancing efficiency and ensuring timely diagnosis.
The objective is to leverage artificial intelligence to enhance safety, reading prioritization, and overall quality of care, especially compared to the current “human only” alternative.
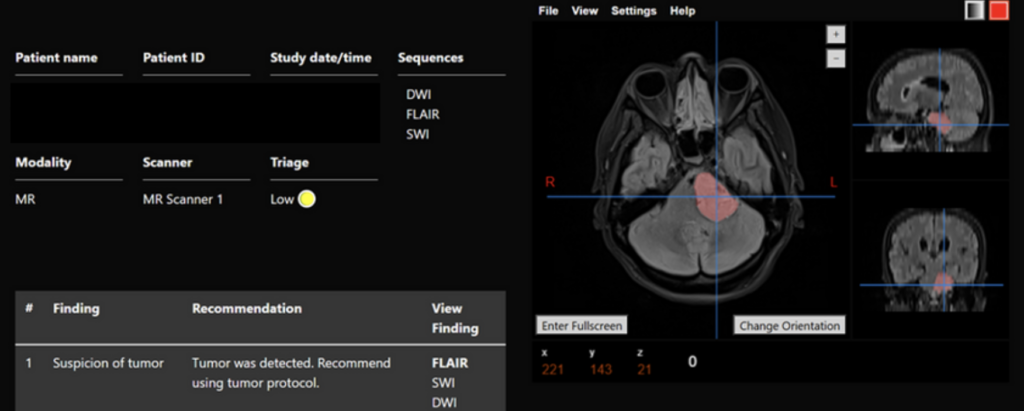
Clinical example of Apollo™ – Outpatient with flagged tumor compressing the brainstem
PERFORMANCE COMPARISON AND CLINICAL IMPACT
Preliminary screening of MRI exams for clinically significant findings is routinely carried out by MR radiographers. In some healthcare institutions, this responsibility is also assigned to radiology trainees, whose levels of experience can vary considerably.
While MR radiographers typically have extensive experience in generating and monitoring MR images and studies, including live quality assurance (QA), they are not diagnostically trained medical professionals. As such, they cannot be formally held accountable for the detection and management of clinically relevant findings.
A recent study evaluating the performance of radiographers and neuroradiologists on abbreviated routine brain MRIs—utilizing a streamlined three-sequence protocol comprising T2-FLAIR, DWI, and SWI/T2* —demonstrated that Apollo exhibits sensitivity comparable to neuroradiologists. Apollo outperformed MR technologists in detecting critical findings that necessitate specialized imaging sequences or subsequent clinical interventions. This highlights the potential of AI to support radiographers and radiologists in identifying clinically significant abnormalities effectively.
Even seasoned radiologists can enhance their diagnostic accuracy through systematic peer review and constructive feedback. Data collected from the onboarding of new radiologists to a teleradiology provider indicate that the implementation of a comprehensive peer review program has the potential to achieve up to a 20-fold reduction in missed findings or erroneous reporting in categories deemed “probably clinically relevant” or “certainly clinically relevant.”
MANAGING REPORTING BACKLOGS
Assuming a prevalence of 10% for clinically significant findings in outpatient brain MRI examinations conducted at an imaging center performing 2,000 scans per month, and a reporting sequence of “first in/first out” – a backlog of one week means an average reporting time of 7 days and about 50 critical cases per week.
Given Apollo’s current performance metrics of approximately 90% sensitivity and 90% specificity, one can anticipate that 45 out of the 50 patients per week with clinically significant findings in the current backlog will be accurately flagged for further evaluation.

Apollo Smart Reading prioritized study list – patients are triaged according to type of finding and local guidelines
REAL-TIME PRIORITIZATION AND WORKFLOW EFFICIENCY
Cases flagged by Apollo™ are prioritized for immediate reading, facilitating timely subsequent actionsthat may be implemented while the patient remains on-site. Although up to 50 cases per week may beflagged as false positives, prioritizing the radiologist’s review and resolution of these cases can be integrated into the same-day workflow. This approach is not expected to materially impact the reporting times for cases with clinically significant findings, thereby maintaining the overall efficiency of the diagnostic process.
*https://www.healthdata.org/sites/default/files/2024-05/GBD_2021_Booklet_FINAL_2024.05.16.pdf
*Kaining Sheng, Silvia Ingala, Jonathan Frederik Carlsen et al. Can AI support on-the-fly brain MRI scan protocol adaptation? RSNA 2024. Pending publication.
*Smart Priority, Smart Protocol and Smart Reading are features of Cerebriu Apollo 2.1.2 (2-2031-5) Cerebriu Apollo is regulated as a medical device in the EU. This product conforms to Medical Device Directive 93/42/EEC and is covered by MDR 2017/745 Article 120. For investigation use only in the US.
DOC ID 2-4100-1